Mechanisms underlying bone loss during spaceflight ~Elucidation of the regulatory mechanism for expression of an osteoclast differentiation factor, RANKL – Publicly Invited Research 2016-2017
- A02 Shinohara
- A02 Maekawa
- A02 Ohgami
- A02 Nishimura
- A02 Kawano
- A02 Iwase
- A02 Furuichi
- A02 Myung
- A02 Kitamura
| Research Subject | Mechanisms underlying bone loss during spaceflight ~Elucidation of the regulatory mechanism for expression of an osteoclast differentiation factor, RANKL |
|---|---|
| Research Group Leader |

|
| Research Collaborator(s) |
|
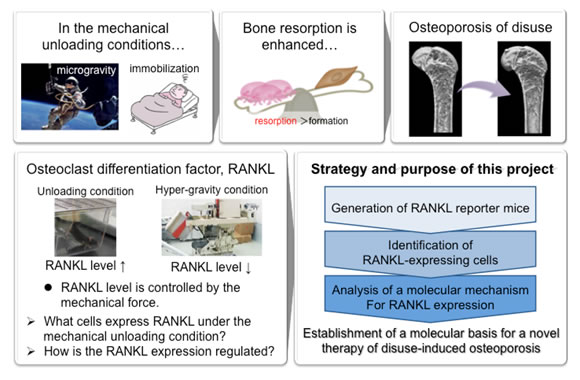
Mechanical loading is a critical factor for maintaining bone tissue homeostasis. Bone-forming osteoblasts and bone-resorbing osteoclasts have critical roles in bone homeostasis. Under mechanical unloading conditions such as microgravity, both bone mass and strength decrease due to enhanced osteoclastic bone resorption, leading to osteoporosis, a bone disease with a high risk of bone fracture. RANKL is a cytokine that plays an essential role in the differentiation of osteoclasts. RANKL is known to be expressed by osteoblasts/osteocytes under physiological conditions, and by T cells, B cells or synovial fibroblasts under certain pathological conditions. However, cells expressing RANKL under mechanical unloading conditions are largely unknown.
In this study, we therefore identify the cells expressing RANKL by using RANKL reporter transgenic mice and also analyze the molecular mechanism for RANKL expression. The findings of this study will provide a molecular basis for future pharmacological therapy of osteoporosis induced by mechanical unloading.